A ground-breaking discovery by a team of researchers from the University of Texas at Austin (UT Austin) captured the world’s attention in December 2021, when they expanded the gene editing possibilities beyond CRISPR-CAS.
CRISPR-CAS is a gene editing mechanism which naturally occurs in bacteria. Discovery of this molecular system led to the winning the 2020 Nobel Prize in Chemistry by the scientists involved. The CRISPR-CAS system can simply be explained as genetic scissors which can cut DNA at specific points. In bacteria it is employed as a protective mechanism against viruses, where their ability to infect cells is destroyed by identifying and cleaving viral DNA. In 2012, scientists Emmanuelle Charpentier and Jennifer A. Doudna proved that these genetic scissors can be manipulated to cut any DNA molecule at a predetermined site. Thereby, specific genetic alterations can be made; cutting out unwanted genes and/or inserting genes or gene clusters with a desired function. This is where CASTs or “CRISPR-associated transposons” come in. CASTs refer to clusters of genes which can insert themselves into an organism’s genome using CRISPR. Thus, an entire gene or relatively large DNA sequence can be inserted into a genome, enabling a wider range of gene editing.
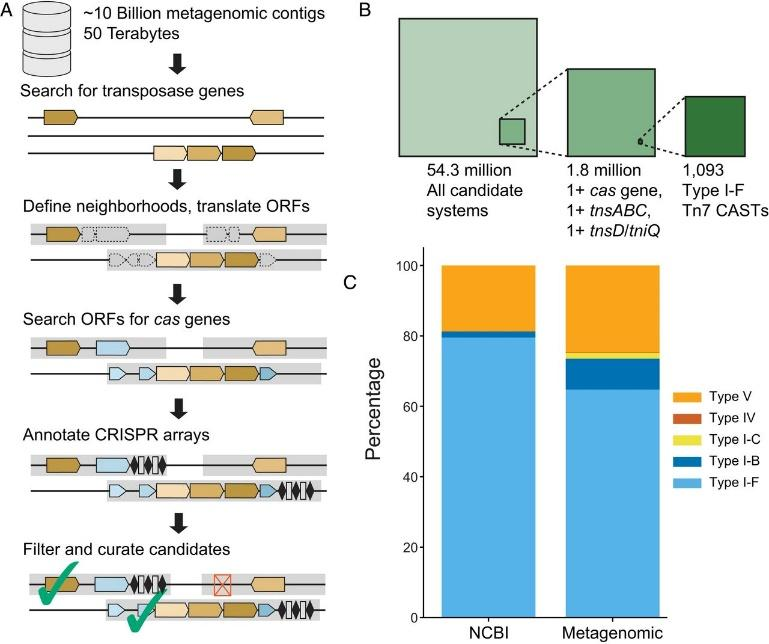
The group at University of Texas, led by Ilya Finkelstein and Claus Wilke revealed that there is possibly a far greater number of gene clusters (CASTs) that can insert themselves into different places in an organism’s genome using CRISPR than initially postulated. After an extensive bioinformatics-based search, their findings raised the number of likely CASTs from 12 to almost 1500.
This discovery expands the research field under molecular biosciences in relation to healthcare and agriculture. In terms of therapeutics, this opens the door to treat multiple-gene disorders and disease conditions. Some of the discovered CASTs have been experimentally verified. However, the long-term challenge remains; to make these systems work accurately in our cells, to achieve deserved genetic changes.
References
Press release: The Nobel Prize in Chemistry 2020 – NobelPrize.org. (n.d.). Retrieved December 7, 2021, from https://www.nobelprize.org/prizes/chemistry/2020/press-release/
Rybarski, J. R., Hu, K., Hill, A. M., Wilke, C. O., & Finkelstein, I. J. (2021). Metagenomic discovery of CRISPR-associated transposons. Proceedings of the National Academy of Sciences, 118(49), e2112279118. https://doi.org/10.1073/PNAS.2112279118
Cover image – https://news.utexas.edu/2020/09/08/matching-crispr-to-the-job-improves-the-safety-efficiency-of-the-gene-editing-tool/
Story By
Nadeeshani Ekanayaka
Associate Member – IOB